More than half (57%) of U.S. adults believe that GM foods are generally unsafe to eat.
A large majority (82%) [of American consumers] support mandatory labels on GMOs, but curiously about the same amount (80%) also support mandatory labels on foods containing DNA.
It is fair to say that discussions about using genetically engineered (GE) crops and animals in American agriculture involve a tremendous amount of misinformation and half-truths. There are vocal advocates for both sides. However, most consumers know little about the role of GE crops in our food supply. That may be why Congress (after being lobbied by industry representatives) stipulated in the 2017 Appropriations Act that the Food and Drug Administration (FDA), in coordination with the U.S. Department of Agriculture, spend $3 million to educate consumers about agricultural biotechnology and biotechnology-derived food products and animal feed. According to the FDA, the goal of its initiative is to provide consumer outreach and education through publication and distribution of science-based educational information on the environmental, nutritional, food safety, economic, and humanitarian impacts of agricultural biotechnology.

To help determine how to implement the initiative, the FDA is seeking public input on what topics to cover and how best to reach consumers. It is unreasonable to believe that with the enormous amount of misinformation out there, the FDA can educate most consumers with information about agricultural biotechnology and its safety, benefits, and consequences with just $3 million. Instead, the FDA should concentrate on providing accurate, neutral, and scientifically-proven information on topics that fall within its areas of expertise to key groups: journalists, nutritionists, doctors, academics, and opinion leaders. Those influencers can then spread that information to consumers.
Safety of foods made from GE crops
The most important topic for the FDA s initiative to focus on is the safety of GE foods. According to numerous national polls, many consumers believe that GE foods and ingredients are not safe to eat. In 2015, for example, the Pew Research Center found that only 37 percent of American adults agreed that it was safe to eat genetically modified (GM) food while 57 percent said that it wasn t. That contrasted with its poll of scientists who are members of the American Association for the Advancement of Science. Eighty-eight percent of them said it was safe to eat GM foods. In a 2016 poll by the Annenberg Public Policy Center, only 24 percent of Americans agreed with the statement that scientists have established that [g]enetically modified foods on the market in the U.S. are as safe as the conventionally grown varieties of the same crop. Another 24 percent thought that statement was false, while 47 percent said they thought that scientists are not sure.
Americans viewpoints in those polls are not consistent with the worldwide scientific consensus that foods and ingredients from currently grown GE crops are as safe as the same foods from non-GE crops. The FDA states on its website that GE plant varieties marketed to date are as safe as comparable, non-GE foods. And after thoroughly reviewing all the available evidence on GE crop safety, a National Academy of Sciences report concluded that no differences have been found that implicate a higher risk to human health and safety from these GE foods than from their non-GE counterparts. That same conclusion has been reached by other respected scientific and regulatory bodies, including the European Commission and the World Health Organization.
Given this disconnect between the facts and consumer opinions, the FDA should focus on providing safety information about GE foods and ingredients. Discussing food safety is within the FDA s area of expertise. The FDA can explain the international safety tests that are required and how regulators independently evaluate safety data before GE food products are allowed to enter the food supply. In addition, the FDA has reviewed safety data from all currently grown GE crops and can speak about that review. FDA s statements on the safety of GE foods would be more authoritative, however, if the FDA had a mandatory pre-market approval process instead of its current voluntary consultation process.
FDA should focus on genetic engineering, genes and DNA
The second, related area that the FDA s initiative should focus on is explaining the science behind genetic engineering and how GE crops end up in our food. In a 2016 poll by the Annenberg Public Policy Center, nearly two-thirds of Americans rated their understanding of GMOs as poor or fair. Similarly, a 2013 poll by Rutgers University found that 54 percent of respondents characterized their knowledge about GMOs as very little or nothing at all.
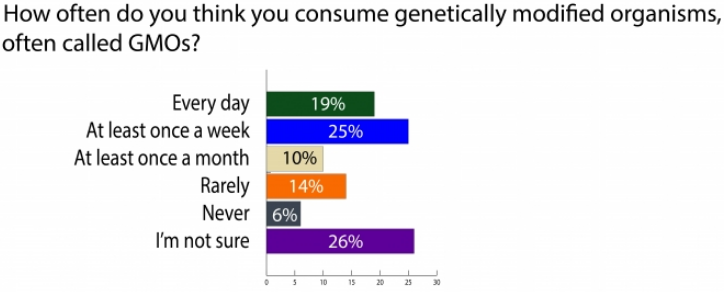
A more recent poll by Michigan State University (MSU) found that when consumers are asked about how often they consume GMOs, 19 percent said every day, 25 percent said at least once a week, 10 percent said at least once a month, and 26 percent said they weren t sure. The reality is that most consumers encounter foods or ingredients made from GE crops daily. What s more, 37 percent found the following statement to be true: [g]enetically modified foods have genes and non-genetically modified foods do not. In fact, that statement is false because all foods that were living have genes, which are composed of sequences of DNA. In fact, several different polls have found overwhelming support for mandatory labeling of foods that contain DNA, which is found in every living organism, including fruits, vegetables, and animals. Clearly, many Americans know little about genetics and genetic engineering.
The FDA is well positioned to provide educational materials on (1) how scientists develop GE crops and animals, (2) which crops and animals have GE varieties on the market, and (3) the types of foods and ingredients made from those GE varieties. FDA can also develop educational materials to explain DNA and genes and what food products they are found in. Finally, to put GE into proper context, the FDA can explain other methods of breeding.
The FDA has scheduled two public meetings to collect comments on its initiative one on Nov. 7 in Charlotte, NC, and one on Nov. 14 in San Francisco, CA. Anyone who lives in those areas should attend those public meetings and provide FDA with their thoughts on which topics FDA should address in its initiative. The three questions FDA is asking commenters to discuss are:
1) What are the specific topics, questions, or other information that consumers would find most useful, and why?
2) Currently, how and from where do consumers most often receive information on this subject?
3) How can the FDA (in coordination with USDA) best reach consumers with science-based educational information on this subject?
If you are unable to attend either meeting, the FDA is accepting written comments through Nov. 17. The more people who tell the FDA to focus its initiative on just a few topics within its expertise, the more likely the initiative can positively affect the national debate on GE foods.
